Cyclic GMP-AMP synthase (cGAS) plays a pivotal role in the cGAS-STING pathway as a DNA sensor that binds to double-stranded DNA (dsDNA) and subsequently induces type I interferon expression, thereby contributing significantly to the innate immune response. Dysregulation of intracellular cGAS has been strongly implicated in a number of diseases, and understanding the mechanisms that control cGAS activity is crucial for developing new strategies to regulate cGAS phase separation and signaling. Several human and viral proteins have been identified that enhance or inhibit cGAS activity. However, due to the lack of quantitative analysis of the interactions between these accessory proteins, cGAS and dsDNA, the molecular logic behind their regulatory mechanisms remains unclear.
On 2nd April 2025, Chunlai Chen's group from the School of Life Sciences at Tsinghua University published a study in Communications Biology entitled "Rational design of chemical- and light-inducible cGAS activation based on mechanistic insights." The study used highly sensitive dual-color fluorescence cross-correlation spectroscopy (dcFCCS) to quantitatively investigate the binding affinities between cGAS, dsDNA and accessory proteins, and revealed that the binding strength between cGAS and accessory proteins is the key factor affecting cGAS phase separation and enzymatic activity. Guided by the new mechanistic insights, the researchers developed chemically and light-inducible cGAS phase separation and signaling regulation systems in test tubes and in living cells.
The proteins G3BP1 and PCBP1 were found to promote cGAS phase separation and enhance its activity, whereas the viral tegument proteins ORF9, ORF52 and VP22 exhibited inhibitory effects. Quantitative dcFCCS characterization showed that inhibitory accessory proteins significantly decreased the hydrodynamic radii of condensates and increased the critical concentrations for phase separation, whereas promoting accessory proteins increased the hydrodynamic radii of condensates and decreased the critical concentrations for phase separation. Further experiments showed significant differences in the binding affinities between these two types of proteins and cGAS. The promoting proteins have relatively strong binding affinities with cGAS. They are able to pre-bind to cGAS to form primary condensates, which can effectively promote the binding and phase separation of cGAS and dsDNA to enhance the enzymatic activity of cGAS. In contrast, viral tegument proteins, which have been shown to inhibit the phase separation and enzymatic activity of cGAS, exhibit weak interactions with cGAS, accompanied by stronger binding to dsDNA than to cGAS.
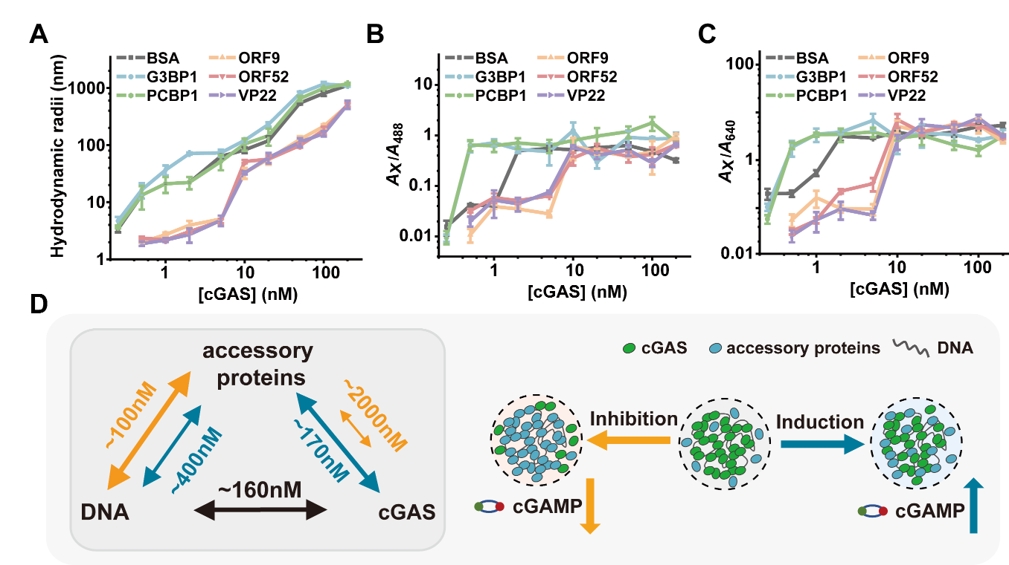
Figure 1. Mechanisms of accessory proteins regulates cGAS phase separation.
Based on the mechanistic insights, the research team proposed to regulate cGAS phase separation and activity by modulating the interactions between cGAS and accessory proteins. The chemical compound rapamycin can induce interactions between the protein domains FKBP and FRB. The researchers used rapamycin to regulate the affinity between FKBP proteins and cGAS-FRB, enabling cGAS to form droplet-like structures within two minutes. In addition, this chemical-inducible system successfully induced rapid phase separation of cGAS in living cells within 5-15 minutes and significantly enhanced its activity.
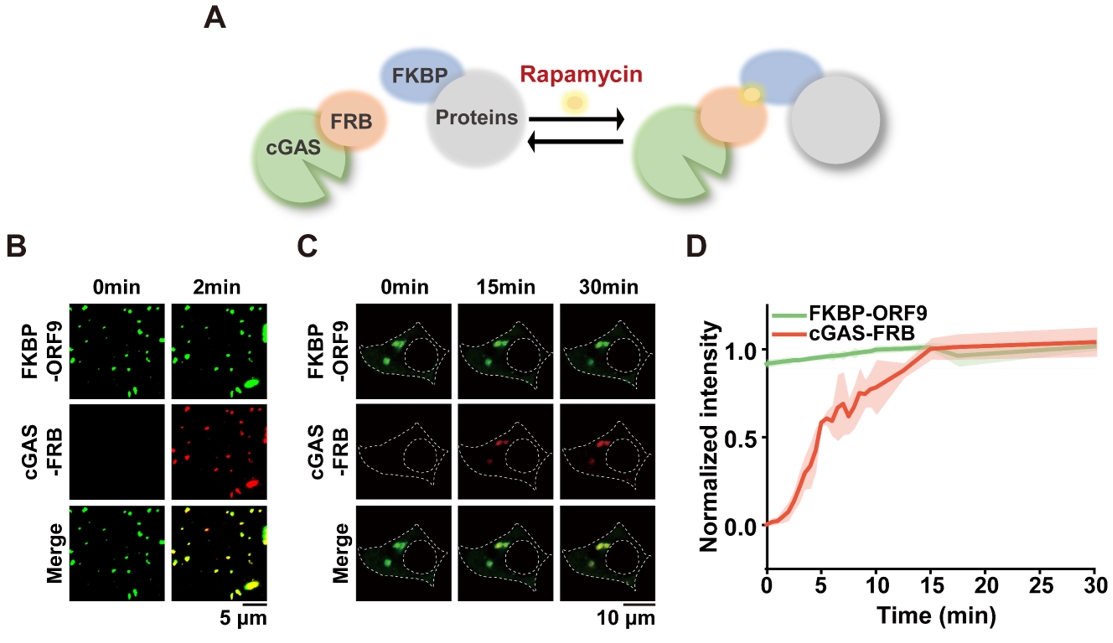
Figure 2. Chemical-inducible cGAS phase separation regulation.
Light-inducible approaches tend to be more spatially and temporally precise than chemical-inducible approaches. The researchers used blue light irradiation to induce pMag-nMagHigh1 dimerization, thereby increasing the affinity between nMagHigh1 proteins and cGAS-pMag, which rapidly induced cGAS phase separation. In living cells, this light-inducible system reversibly regulated cGAS phase separation, with cGAS aggregates gradually dissociating after blue light termination.
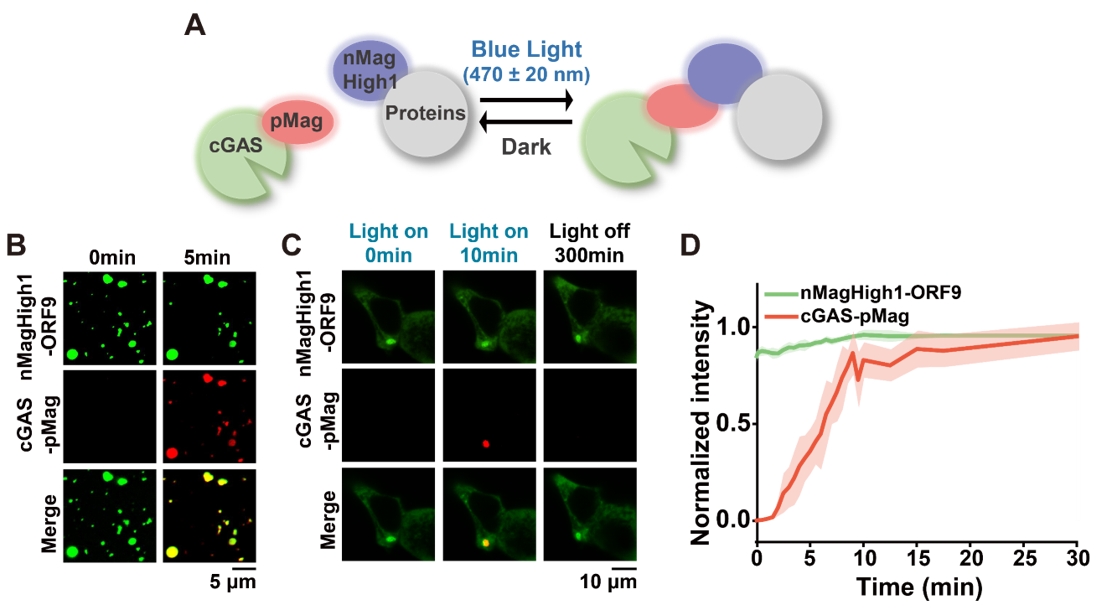
Figure 3. Light-inducible cGAS phase separation regulation.
The chemical- and light-inducible systems developed in this study minimally affect the valency of molecular interactions, providing tools with excellent spatial and temporal precision for investigating cGAS phase separation and immune signaling. Moreover, the mechanistic insights offer guidance and new avenues to manipulate multi-component, especially protein-nucleic acid, phase separation systems in cells with high temporal and spatial specificity.
The research was funded by the National Natural Science Foundation of China, the Joint Research Fund between the National Natural Science Foundation of China and the Hong Kong Research Grants Council, the National Key Research and Development Program of China, the Beijing Frontier Research Center for Biological Structure, and the State Key Laboratory of Membrane Biology.
Link to the article:
https://www.nature.com/articles/s42003-025-07892-5
Editor: Li Han

